The elements present in the third period contain d orbitals in addition to s and p orbitals. The energy of the 3d orbitals are comparable to the energy of the `3s` and `3p` orbitals. The energy of `3d` orbitals are also comparable to those of `4s` and `4p` orbitals. As a consequence the hybridisation involving either `3s, 3p` and `3d` or `3d, 4s` and `4p` is possible. However, since the difference in energies of 3p and 4s orbitals is significant, no hybridisation involving `3p, 3d` and `4s` orbitals is possible.
The important hybridisation schemes involving s, p and d orbitals are summarised
below:
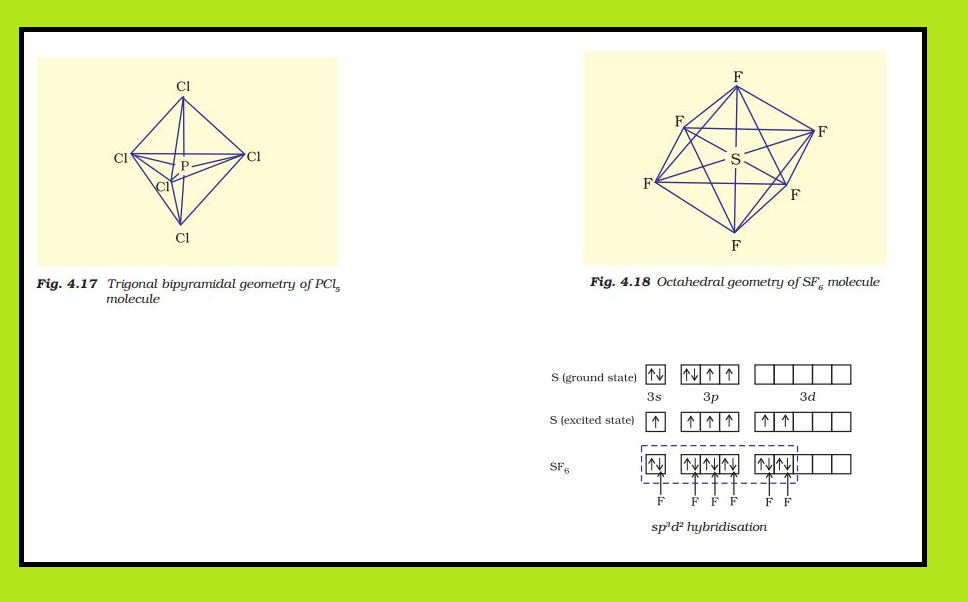
(i) Formation of `PCl_5` (`sp^3d` hybridisation): The ground state and the excited state outer
electronic configurations of phosphorus (Z=15) are represented below.
Now the five orbitals (i.e., one s, three p and one d orbitals) are available for hybridisation to yield a set of five sp3d hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal as depicted in the Fig.
It should be noted that all the bond angles in trigonal bipyramidal geometry are not equivalent. In `PCl_5` the five `sp^3d` orbitals of phosphorus overlap with the singly occupied p orbitals of chlorine atoms to form five `P–Cl` sigma bonds. Three `P–Cl` bond lie in one plane and make an angle of `120°` with each other; these bonds are termed as equatorial bonds. The remaining two `P–Cl` bonds–one lying above and the other lying below the equatorial plane, make an angle of 90° with the plane. These bonds are called axial bonds. As the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than the equatorial bonds; which makes `PCl_5` molecule more reactive.
(ii) Formation of `SF_6` (`sp^3d^2` hybridisation) : In `SF_6` the central sulphur atom has the ground state outer electronic configuration `3s^2 3p^4`. In the exited state the available six orbitals i.e., one s, three p and two d are singly occupied by electrons. These orbitals hybridise to form six new `sp^3d^2` hybrid orbitals, which are projected towards the six corners of a regular octahedron in `SF_6`. These six `sp^3d^2`
hybrid orbitals overlap with singly occupied orbitals of fluorine atoms to form six `S–F` sigma bonds. Thus `SF_6` molecule has a regular octahedral geometry as shown in Fig.
The elements present in the third period contain d orbitals in addition to s and p orbitals. The energy of the 3d orbitals are comparable to the energy of the `3s` and `3p` orbitals. The energy of `3d` orbitals are also comparable to those of `4s` and `4p` orbitals. As a consequence the hybridisation involving either `3s, 3p` and `3d` or `3d, 4s` and `4p` is possible. However, since the difference in energies of 3p and 4s orbitals is significant, no hybridisation involving `3p, 3d` and `4s` orbitals is possible.
The important hybridisation schemes involving s, p and d orbitals are summarised
below:
(i) Formation of `PCl_5` (`sp^3d` hybridisation): The ground state and the excited state outer
electronic configurations of phosphorus (Z=15) are represented below.
Now the five orbitals (i.e., one s, three p and one d orbitals) are available for hybridisation to yield a set of five sp3d hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal as depicted in the Fig.
It should be noted that all the bond angles in trigonal bipyramidal geometry are not equivalent. In `PCl_5` the five `sp^3d` orbitals of phosphorus overlap with the singly occupied p orbitals of chlorine atoms to form five `P–Cl` sigma bonds. Three `P–Cl` bond lie in one plane and make an angle of `120°` with each other; these bonds are termed as equatorial bonds. The remaining two `P–Cl` bonds–one lying above and the other lying below the equatorial plane, make an angle of 90° with the plane. These bonds are called axial bonds. As the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than the equatorial bonds; which makes `PCl_5` molecule more reactive.
(ii) Formation of `SF_6` (`sp^3d^2` hybridisation) : In `SF_6` the central sulphur atom has the ground state outer electronic configuration `3s^2 3p^4`. In the exited state the available six orbitals i.e., one s, three p and two d are singly occupied by electrons. These orbitals hybridise to form six new `sp^3d^2` hybrid orbitals, which are projected towards the six corners of a regular octahedron in `SF_6`. These six `sp^3d^2`
hybrid orbitals overlap with singly occupied orbitals of fluorine atoms to form six `S–F` sigma bonds. Thus `SF_6` molecule has a regular octahedral geometry as shown in Fig.